Usp Catalog
Usp Catalog - Find out what's new at usp, including reference standards, monographs, impurities, and more. Authorized distributor for the us pharmacopeia. Find the current lots and lot designations of usp reference. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the. A complete listing of usp reference standards along with lot, date, and ordering information is available online in downloadable xls and pdf formats. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Browse the quality solution sheets (qss) to access all the relevant. Find excipients, foods, general chapters, complex generics and more. Catalog # item description catalog # item description. Our pai portfolio now has over 700 impurity analytical reference materials covering over 150 active. Find excipients, foods, general chapters, complex generics and more. Catalog # item description catalog # item description. Find the usp reference standards catalog, web. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Please ensure that there are no duplicate/inactive items across multiple lines. Enter each individual item numbers and the quantities for each. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Over 200 new pharmaceutical analytical impurities have been added to our catalog. Browse the quality solution sheets (qss) to access all the relevant. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Find the current lots and lot designations of usp reference. Our pai portfolio now has over 700 impurity analytical reference materials covering over 150 active. Usp nf online + usp education bundle. Usp offers more than 3,500. Find the usp reference standards catalog, web. Learn about the usp reference standards, their selection, testing, distribution, and use in pharmacopeial assays and tests. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Over 200 new pharmaceutical analytical impurities have been added to our catalog. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Learn about the usp reference standards,. Learn about the usp reference standards, their selection, testing, distribution, and use in pharmacopeial assays and tests. Our pai portfolio now has over 700 impurity analytical reference materials covering over 150 active. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Search and order from the complete catalog of official usp reference standards. Usp. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the. Find excipients, foods, general chapters, complex generics and more. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Learn about the usp reference. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Search and order from the complete catalog of official usp reference standards. A complete listing of usp reference standards along with lot, date, and ordering information is available online in downloadable xls and pdf formats. Find the usp reference standards catalog, web. Browse the quality. Usp nf online + usp education bundle. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. A complete listing of usp reference standards along with lot, date, and ordering information is available online in downloadable xls and pdf formats. Find excipients, foods, general chapters, complex generics. Search and order from the complete catalog of official usp reference standards. Find out what's new at usp, including reference standards, monographs, impurities, and more. Authorized distributor for the us pharmacopeia. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients,. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary supplements, compendial reagents and. Browse and order usp reference standards for pharmaceutical, biologic, dietary supplement and herbal products. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the. Learn. Enter each individual item numbers and the quantities for each. Find the usp reference standards catalog, web. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Usp offers more than 3,500. Our pai portfolio now has over 700 impurity analytical reference materials covering over 150 active. Authorized distributor for the us pharmacopeia. Usp nf online + usp education bundle. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary supplements, compendial reagents and. Browse the quality solution sheets (qss) to access all the relevant. Find the usp reference standards catalog, web. Please ensure that there are no duplicate/inactive items across multiple lines. Find out what's new at usp, including reference standards, monographs, impurities, and more. Learn about the usp reference standards, their selection, testing, distribution, and use in pharmacopeial assays and tests. Browse and order usp reference standards for pharmaceutical, biologic, dietary supplement and herbal products. Catalog # item description catalog # item description. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Search and order from the complete catalog of official usp reference standards. Over 200 new pharmaceutical analytical impurities have been added to our catalog. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the.USP Structural Connectors Catalog USA by Ram Tool Construction Supply
USPStructuralConnectorsCatalogCanada.pdf Corrosion Zinc
MiTek Launches USP Catalog App Version 2.2.0 for Windows, Apple, and
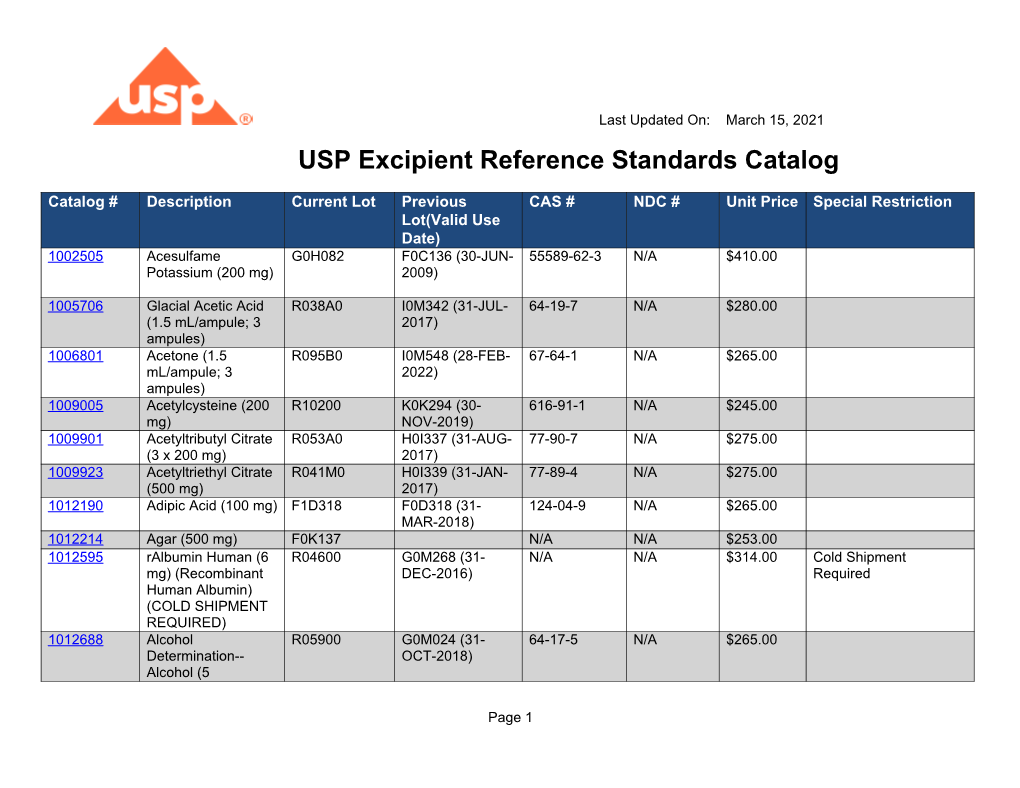
USP Excipient Reference Standards Catalog DocsLib
Underlayment Specialties Plus (USP), Catalogs, Product Catalog ARCAT
USP Structural Connectors Catalog USA by Ram Tool Construction Supply
(PDF) Sac uspcatalogver.1
2018 Online Catalog USP Connectors USA by of Florida
Underlayment Specialties Plus (USP), Catalogs, Product Catalog ARCAT
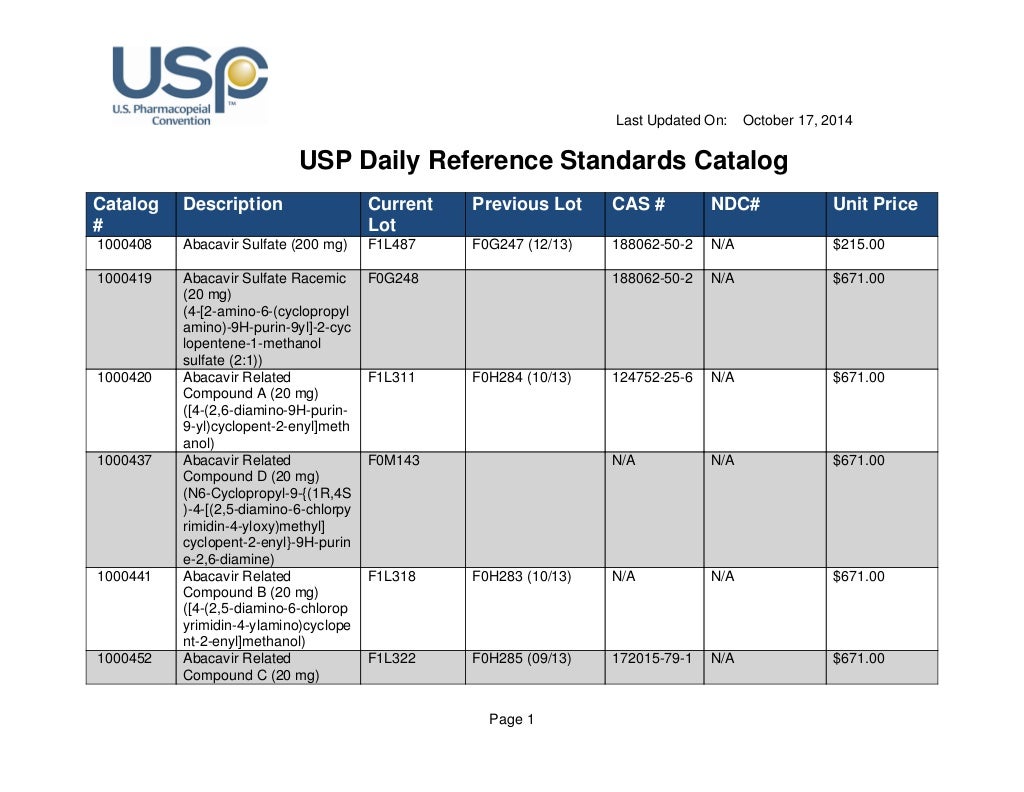
Usp daily reference standards catalog
Enter Each Individual Item Numbers And The Quantities For Each.
A Complete Listing Of Usp Reference Standards Along With Lot, Date, And Ordering Information Is Available Online In Downloadable Xls And Pdf Formats.
Find The Current Lots And Lot Designations Of Usp Reference.
Find Excipients, Foods, General Chapters, Complex Generics And More.
Related Post: